Multiple Functions of Creatine Kinase for Cellular Energetics: a Scientific Rationale for Creatine Supplementation
This
article is protected by
copyright and is intended for scientists,
doctors
and patients and
for
non-commercial use only.
For any kind of
reproduction
for commercial purposes a written permission is
required
which may be obtained by:
Dr. Theo Wallimann, Prof. Emeritus, Institut für Zellbiologie, ETH Zürich-Hönggerberg, CH-8093 Zürich
Privat: Schürmattstrasse 23, CH-8962 Bergdietikon, Tel.: +41-(0)44-740-70-47, Fax: +41-(0)44-741-30-08
E-mail: theo.wallimann@cell.biol.ethz.ch
Internet: http://www.mhs.biol.ethz.ch/about-us/emeriti-formermembers/wallimann.html
Abstract
Creatine kinase (CK)
isoenzymes are found in cells with intermittently high energy
requirements. They are specifically located at places of energy
demand and energy production and are linked by a
phosphocreatine/creatine (PCr/Cr) circuit. Cytosolic CK, in close
conjunction with Ca2+-pumps, plays a crucial role for the energetics
of Ca2+-homeostasis. Mitochondrial Mi-CK, a cuboidal-shaped octamer
with a central channel, binds and cross-links mitochondrial membranes
and forms a functionally coupled microcompartment with porin (VDAC)
and adenine nucleotide translocase (ANT) for vectorial export of PCr
into the cytosol. The CK system is regulated by AMP-activated protein
kinase via the ATP/AMP-, as well as the PCr/Cr ratio. Mi-CK
stabilizes and cross-links cristae- or inner/outer membranes to form
parallel membrane stacks and, if overexpressed due to
creatine-depletion or cellular energy stress, forms those crystalline
intramitochondrial inclusions often seen as hallmarks in
mitochondrial cytopathy patients. Mi-CK is a prime target for free
radical damage by peroxynitrite. Mi-CK octamers, together with CK
substrates have a marked stabilizing and protective effect against
mitochondrial permeability transition pore (PTP) opening, thus
providing a rationale for creatine supplementation of patients with
neuromuscular and neurodegenerative diseases. In addition to the well
documented improvement of high-intensity intermittent exercise
performance after creatine supplementation, recent results seem to
indicate that creatine supplementation may also favourably affect
long-endurance exercise. Chronic high-dose creatine ingestion,
however, was shown to down-regulate the expression and/or
accumulation of creatine transporter polypeptides in skeletal muscle
of the rat. Thus, a one month pause, after three month of creatine
supplementation, as suggested earlier, seems a reasonable
advise.
key words and abbreviations:
creatine kinase (CK), creatine (Cr), phosphocreatine (PCr), PCr-shuttle, energetics of Ca2+-homeostasis, CK null-mutant transgenic mice, mitochondrial creatine kinase (Mi-CK), intramitochondrial inclusions, mitochondrial myopathies, AMP-activated protein kinase (AMPK), mitochondrial permeability transition (MTP), peroxynitrite PN), porin (P), adenine nucleotide translocase (ANT), cell- and neuroprotective effects of creatine, creatine supplementation, neuromuscular diseases, short-term physical performance, high-intensity-long-endurance exercise, creatine transporter (CreaT)
The creatine kinase / phospho-creatine circuit
The enzyme creatine kinase (CK), catalyzing the reversible
transfer of the N-phosphoryl group from phosphocreatine (PCr) to ADP
to regenerate ATP, plays a key role in the energy homeostasis of
cells with intermittently high, fluctuating energy requirements, e.g.
skeletal and cardiac muscle, neurons, photoreceptors, spermatozoa and
electrocytes. Cytosolic CK isoenzyme(s) (MM-, MB- and BB-CK) are
always co-expressed in a tissue-specific fashion together with a
mitochondrial isoform. Using biochemical fractionation and in situ
localization, one was able to show that the CK isoenzymes, earlier
considered to be strictly soluble, are in fact compartmentalized
subcellularly and coupled functionally and/or structurally either to
sites of energy production (glycolysis and mitochondria) or energy
consumption (cellular ATPases, such as the acto-myosin ATPase and
SR-Ca2+-ATPase). Thus they form an intricate, highly regulated energy
distribution network, the so-called PCr-circuit or PCr-shuttle
(Figure 1, for review see [1] and the special volumes of Mol. Cell
Biochem. 133/134, 1994, and 184, 1998).
This
non-equilibrium energy transport model has been challenged, based
upon global 31P-NMR experiments, measuring CK-mediated flux in
muscles at different work-loads [2,3]. The conclusions reached by
these authors were i) that the CK system is in equilibrium with the
substrates, behaving like a solution of well-mixed enzymes, ii) that
effects of compartmentation were negligible with respect to total
cellular bioenergetics and iii) that thermodynamic characteristics of
the cytosol could be predicted as if the CK metabolites were freely
mixing in solution. However, based on the organizational principles
of sarcomeric muscle, as well as on our findings concerning the
highly structured subcellular CK-compartments, this interpretation
seemed rather unlikely and thus has been questionned [4]. In support
of this, 31P-NMR CK-flux measurements with transgenic mice showing
graded reductions of MM-CK expression in their muscles, revealed a
strikingly unexpected, “anomalous” CK-flux behaviour [5].
These results indicate that some flux through CK, presumably bound
CK, and possibly also some PCr and/or ATP, are NMR-invisible or
otherwise not amenable to this analysis [4,6]. In the meantime, more
evidence from NMR-measurements [7,8,9,10], as well as from recent in
vivo 14[C]Cr-tracer studies [11], is accumulating in favour of
compartmentation of the CK system and for the existence of different
pools of CK substrates. As a matter of fact, it has now become clear
that in muscle, Cr and PCr molecules do not tumble freely, but
display partial orientational ordering, which is in contrast to what
is expected for small molecules dissolved in water [7]. Furthermore,
31P-NMR saturation transfer experiments with sea-urchin spermatozoa
show that the CK-flux increases by a factor of 10-20 upon sperm
activation [12]. These specialized sperm cells derive their energy
for motility entirely from fatty oxidation within the single large
mitochondrion located just behind the sperm head, from where PCr is
diffusing along the 50 µm long sperm tail to fuel the
dynein/tubulin ATPase. It is obvious that in these polar, elongated
cells, the diffusional limitation of ADP is the key limiting factor
with respect to high-energy phosphate provision [13]. Also in support
of the PCr-shuttle model, the calculated diffusional flux of ADP in
these sperm cells is by 2 and 3 orders of magnitude smaller than
those of ATP and PCr, respectively [13].
In conclusion, it
becomes obvious that calculations of free cellular [ADP] by using
global [ATP] and [PCr], determined by in vivo 31P-NMR, together with
the CK equilibrium constant, may be valid only in certain limited
cases, e.g. in fast twitch glycolytic white muscle fibres, where the
buffer function of CK by far prevails the transport function and
where the flux through the CK reaction at rest and during high work
load are higher by a factor of 100 and 20, respectively, than the
total cellular ATPase turnover at these respective states. In cases
where the transport function of the CK prevails, e.g. oxidative
tissues or in polar cells (sea urchin sperms) with high
concentrations of Mi-CK, local [ADP] and [ATP] levels, e.g. in the
mitochondrial intermembrane space or near CK-ATPase complexes, may
differ by orders of magnitude compared to the bulk concentrations
calculated from the CK equilibrium constant. Considering the
complications of subcellular compartmentation of CK isoenzymes in a
cell, where after activation, some CK will work in the forward and
some in the reverse direction, the interpretation of global CK flux
measurements may also represent a rather difficult endeavour.
The importance of creatine kinase for calcium homeostasis and muscle contraction:
Transgenic
CK(-/-) double knock-out mice show significantly increased relaxation
times of their limb muscles, altered Ca2+-transients in myotubes
after stimulation, as well as remarkable remodelling of the
contractile apparatus with increased numbers of mitochondria and
grossly over-produced tubular SR membranes [14]. The obvious
difficulties of these mice with muscle Ca2+-handling, as the main
phenotype, is in line with biochemical and functional data showing
that some MM-CK is specifically associated with SR membranes [15],
where it is crucial for fueling the energetically highly demanding
Ca2+-ATPase [15,16,17]. The strong dependence of Ca2+ regulation by
the SR on the supply of ATP via endogenous SR-bound has also been
confirmed very recently with mechanically skinned muscle fibers [93].
Thus, depletion of PCr may contribute to impaired SR Ca2+-regulation
known to occur in inteact skeletal muscle under conditions of
fatigue. Therefore, one of the most crucial function of the CK-system
in muscle seems to be related to the energetics of Ca2+-homeostasis
[6].
In addition, some CK is also associated with the
myofibril [1]. The domain responsible for the isoenzyme-specific
binding of MM-CK to the myofibrillar M-band has been localized by an
in situ biochemical approach, using heterologously expressed,
fluorescently labelled site-directed mutants, as well as M/B-CK
chimaeras for diffusion into chemically skinned skeletal muscle
fibers [18]. This M-band interaction domain could be narrowed down to
two “charge-clamps”, symmetrically organized on a exposed
face of each M-CK monomer [80]. Using the same approach to study the
weak MM-CK binding to the myofibrillar I-band, observed by in situ
immunofluorescence localization, we found that MM-CK binding to this
sarcomeric region is mediated by some glycolytic enzymes [19].
AMP-activated protein kinase a ratiometric PCr/Cr energy sensor at last:
According to recent findings, AMP-activated protein kinase (AMPK) is able to bind rather tightly to muscle-type MM-CK and phosphorylate the latter to inhibit its activity to a certain extent. Most surprisingly, it was found by the same authors that AMPK itself is regulated not onlyby the ATP/AMP ratio, but also by the PCr/Cr ratio [20]. This invalidates the long-held dogma that PCr and Cr are metabolically completely inert compounds. Thus, AMPK, as an energy sensor system, could represent the missing link for regulation of adaptive metabolic changes, e.g. after depletion of creatine levels in skeletal and cardiac muscle. Interestingly enough, both the ablation of the muscle-type CK isoenzymes in transgenic animals [14] or the depletion of creatine, the substrate of the CK reaction, after supplementation with β-GPA [50], seem to elicite very similar adaptational effects in skeletal muscle. The activation of AMPK by decreasing PCr/Cr ratios and increasing [AMP], as observed during muscle activation at high work-load would lead to progressively stronger inactivation of cytosolic muscle-type MM-CK [20]. This could very well explain the long-standing enigma why, in muscle, the CK-mediated reaction flux, which can be more than 10-20-fold higher, depending on the muscle type, than the highest ATPase turnover, does not increase with higher workload, but rather tends to decrease instead [78,79].
Mitochondrial creatine kinase for metabolic channeling of high-energy phosphate compounds:
Mitochondrial creatine kinase (Mi-CK) is located in the mitochondrial intermembrane space along the inner membrane, but also at contact sites where inner and outer membranes are in close proximity [1,48]. Mi-CK can directly transphosphorylate intramitochondrially produced ATP into PCr, which subsequently is exported to the cytosol. A well documented role of Mi-CK is the functional coupling of mitochondrial CK to oxidative phosphorylation [21,22]), which facilitates the antiport of ATP versus ADP through the inner membrane via the adenine nucleotide translocator (ANT). In addition, a physical interaction of Mi-CK with outer mitochondrial membrane porin (VDAC) has also been demonstrated [23]. The solved atomic X-ray structure of octameric Mi-CK [24] is consistent with the proposed energy channeling function of this enzyme. Detailed structure/function analyses concerning the molecular physiology, catalytic site and mechanism, octamer/dimer equilibrium, as well as the interaction of Mi-CK with mitochondrial membranes have been published [21,25]. The identical top and bottom faces of the octamer contain putative membrane binding motifs likely to be involved in binding of Mi-CK to mitochondrial membranes. The central 26 » wide channel of the Mi-CK octamer may be of functional significance for the exchange of energy metabolites between mitochondria and cytosol. If Mi-CK would follow a “back door” mechanism by which PCr is be expelled into the central channel of the Mi-CK octamer, as depicted in hypothetical models (see Figs. 6A and 7 in ref. [21]), vectorial transport of PCr from the mitochondrial matrix into the cytosol could be greatly facilitated.
Exquisite sensitivity of Mi-CK to peroxynitrite, effects on cellular calcium homeostasis and linkage to pathological states:
Peroxynitrite (ONOO-, PN), the
product of the reaction between nitrogen monoxide (NO) and the
superoxide anion O2- has been shown to be highly reactive towards
Mi-CK [26]. Recently, a mitochondrial NO synthase isoform has been
discovered [27]. Thus, mitochondria as a notorious source of O2-,
especially after ischemia/reperfusion episodes, additionally produce
PN internally. We have found that Mi-CK in intact mitochondria is a
prime target of inactivation and modification by PN, at
concentrations of PN that are much lower than those needed for
inactivation of mitochondrial respiratory chain enzymes [26]. The
pronounced sensitivity of Mi-CK towards reactive oxygen species
(ROS), especially peroxynitrite, may explain the effects seen after
perturbation of cellular pro-oxidant/antioxidant balance, e.g. after
ischemia/reperfusion. These effects include energy failure,
paralleled by elevated ADP levels and chronic calcium overload due to
inactivation of the CK system. Perfusion of hearts with NO donors
lead to an inhibition of cardiac CK by 65% and a concomitant decrease
in heart contractile reserve [28]. Stimulation of inducible
NO-synthase (NOS), which is indeed increased in vivo in skeletal
muscle biopsies from patients with chronic heart failure [29], also
leads to a NO-dependent depression of cardiac function [30]. Thus, a
correlation between a compromised CK system and energy failure of the
heart becomes obvious.
Most recently, we found that PN is
also affecting the oligomeric state of Mi-CK. PN-treatment of Mi-CK
octamers leads to some dimerisation, whereas treatment of dimeric
Mi-CK with the same reagent prevents reoctamerization of Mi-CK dimers
in a PN-concentration dependent manner [31]. These findings may
explain why in different models of cardiac infarction, one
consistently detects a significantly enhanced proportion of Mi-CK
dimers as compared to in non-infarcted heart tissue [81].
The results that cytosolic CK's, and therefore also SR-bound MM-CK,
which is functionally coupled to the SR-Ca2+-pump [15-17,93], are
also very sensitive to reactive oxygen species (ROS) as well [32,33],
indicate that impairment of the CK system by ROS would severely
disturb cellular Ca2+-handling and homeostasis. As a consquence of
cellular Ca2+-overload, resulting among other factors in a break-down
of mitochondrial membrane potential, mitochondria may release
additional Ca2+ into the cytosol [34], thus aggravating the situation
even more [35]. The interaction of elevated Ca2+-levels and raise in
[ROS] would then lead into a vicious cycle with progressive
inactivation of both Mi-CK and SR-bound MM-CK. Therefore, the
destabilization of cellular energetics by chronic exposure to ROS,
thought to occur in many neuromuscular diseases [36], may finally
lead to apoptosis or cell death, especially in those cells with high
mitochondrial activity. Skeletal muscle and cardiac or neuronal cells
are ideal candidates as chronically elevated Ca2+-levels or
Ca2+-overload has been identified as a major player of cell
destruction [36]. A clear link between chronically elevated
Ca2+-concentration and a calcineurin-dependent signalling pathway,
eventually leading to cardiac hypertrophy and chronic heart failure
has been demonstrated very recently [35]. In accordance with the CK/
Ca2+-connection, in brain, the concentration of CK was found to be
very high in those cells that display high-frequency Ca2+-spiking,
e.g. cerebellar Purkinje neurons, as well as granule and pyramidal
cells of the hippocampus [37]. A most recent finding, showing that in
neurodegenerative diseases, like Alzheimer's disease, CK enzyme
activity is severely reduced and cytosol-membrane partitioning is
aberrant [38], also corroborates the imporant role of the
CK/PCr-system in the energetics of brain pathology.
Involvement of Mi-CK and CK substrates in mitochondrial permeability transition and early apoptosis:
A protein
complex containing ANT and mitochondrial porin has recently been
described to display the characteristics of the mitochondrial
permeability transition pore (MTP) or mega-channel [39]. The physical
interaction and functional coupling of Mi-CK with porin and ANT
indicates an involvement of Mi-CK in the regulation of MTP, since
octameric Mi-CK [1] in this protein complex [23,39,40], plus creatine
or creatine analogues, can delay MTP [41]. This has been demonstrated
by using transgenic mice that express Mi-CK in liver. Since liver of
wild-type animals do not contain this enzyme, but otherwise are
identical, mitochondria from wt livers serve as an ideal control. Our
experiments provided exciting new evidence that Mi-CK is not only
involved in mitochondrial energy transfer and shuttling of
high-energy phosphate, but may also participate directly in
mitochondrial permeability transition (MPT) [41]. The Ca2+-dependent
increase of inner membrane permability to ions and solutes is
dependent on the transmembrane potential difference, matrix pH,
SH-group reactants and is modulated by a variety of effectors.
Cyclosoporin-A turned out to be a very potent inhibitor of MPT [42].
Interestingly, creatine or cyclo-creatine delayed
cyclosporin-A-sensitive swelling and inhibited concomitant increase
of state-4 respiration of mitochondria from Mi-CK-containing
transgenic livers [41]. No comparable effect was seen with control
liver mitochondria that do not contain any CK. This novel
Mi-CK-related phenomenon deserves further attention since it may shed
some new light on the recently observed neuroprotective effects of
creatine and its analogues in animals models [43,44,85].
In
addition, protein complexes, containing octameric Mi-CK, porin and
ANT, could be isolated from detergent solubilized rat brain extracts
[39,40]. After reconstitution into malate-loaded lipid vesicles, the
presence of octameric Mi-CK prevented Ca2+-induced malate release,
which, however, was observed after dimerization of Mi-CK [41]. The
fact that highly purified ANT, functionally reconstituted as ATP/ADP
exchange carrier, displayed a Ca2+-dependent release of internal
substances, while atractyloside or HgCl2, both induced unspecific
pore opening of ANT, indicate that ANT is capable of adopting a
pore-like structure under conditions known to induce MPT [45]. Mi-CK
has been shown to be functionally coupled to ANT (for review see [1,
22, 46, 47] and to form complexes with porin and ANT [40]. Therefore,
it is obvious that Mi-CK octamers could directly affect this
ANT-mediated permeability transiton. Thus, the arrangement of Mi-CK
as an energy channeling unit sandwiched in between porin and ANT and
linking OM and IM together, seems not only important for high-energy
phosphate conversion and transport (see Figure 1), but the molecule
may also act as a protective regulatory component of the permeability
transition complex. Depending on the cellular energy state and
intracellular [Ca2+], octameric Mi-CK may prevent MTP [48], an early
event in the execution of apoptosis [49] in cells with high energy
demands, thus sparing the cells from- or delaying cell death. On the
other hand, dimerization of the Mi-CK octamer may allow the ANT to
switch to its MTP-like state [48], eventually leading to
apoptosis.
Enhancement of physical performance by creatine supplementation:
The CK/PCr system is now
recognized as an important metabolic regulator during health and
disease. Creatine, synthesized in part by the body, but also ingested
by food, especially meat and fish (for review see [50]), is taken up
into cells by a creatine transporter (CreaT) (for review see [51]).
Creatine supplementation in humans leads to an increase in
intracellular [Cr] and [PCr], concomitantly improving anaerobic
performance of muscle [52,53], shortens muscle relaxation time [83],
increases fat free- or lean body mass [94] as well as the
cross-sectional area (fiber diameter) of all muscle fiber types [93].
In addition, creatine seems to improve recovery after exhaustive
excercise [54] (for review see [55,56]). One could show that creatine
supplementation may also have beneficial effects for high-intensity,
aerobic long-endurance exercise [57]. In a double-blinded
placebo-controlled study, 20 highly trained top athletes were
subjected at 1?650 meters above sea level (in Davos, Switzerland) to
a series of spiro-ergometric short- and long-term performance tests
before and after 10 days of supplementation with 3x3.3 g of Cr per
day. In accordance with earlier studies, short performance and
maximal work output were both improved by approx. 30 Watt. In a 1
hour spiro-ergometric test at 85% power output of the individually
determined anaerobic threshold, the Cr group was able to perform,
after Cr supplementation, at the same level of exercise with a
significantly lower heart rate (-8.4 beats/min) than before Cr
intake. In this group, lactate levels were lower by 0.48 mM/l and
Borg scale numbers by 1.35 points. These effects were not observed in
the controls. Ventilation, VO2 and respiratory quotient (RQ) were
basically unchanged [57]. The effects of Cr on endurance performance
seem to be due to increased efficiency of energy utilization by heart
and skeletal muscle which may be related to the involvement of CK in
the energetics of Ca2+-homeostasis. As a consequence of creatine
supplementation, the elevated cellular PCr level is likely to
increase the supply of the SR-Ca2+-ATPase with high-energy phosphates
via the coupled CK reaction and thus would also increase the
efficiency of Ca2+-pumping and delay impaired Ca2+-regulation known
to occur under conditions of fatigue [93]. During long-endurance
exercise, this process consumes a significant proportion of the
available bioenergy. In addition, Cr-stimulated respiration and
enhanced resynthesis of PCr after creatine ingestion [54] and/or the
recently discovered control of AMP-activated protein kinase by the
PCr/Cr ratio [20] and its effects on CK and lipid metabolism in
general [20] could be important factors leading to the observed
improvement of aerobic exercise described above.
An
important new aspect of creatine supplementation was descovered only
recently, that is, creatine supplementation in combination with
carbohydrate loading after submaximal glycogen-depleting exercise not
only markedly improves Cr uptake, but also increases glycogen
accumulation in human muslcle [96]. Thus, the highly elevated levels
of glycogen reached after combined carbohydrate and creatine loading
after glycogen-depleting exercise may, of course, also add to the
positive effect of creatine supplementation on long-endurance
exercise [57].
Down-regulation of the creatine transporter after chronic creatine ingestion:
The creatine transporter
(CreaT), responsible for the uptake of creatine into a variety of
tissues and cells, was detected in rat skeletal and cardiac muscle,
cerebellum, forebrain and kidney. Two polypeptides with an apparent
Mr of 70 kDa and 55 kDa were always recognized by both of our
specific polyclonal antibodies directed against synthetic peptides of
either the NH2- or the COOH-terminus of CreaT, indicating a high
degree of homology between the two proteins [51]. In contrast to
published data obtained by Northern blot analysis, suggesting a
complete absence of CreaT mRNA message in liver, we could clearly
detect both CreaT polypeptides also in rat liver and hepatocyte
lysates. In support of this, cultured hepatocytes show an endogenous
CreaT activity which is antagonized by the creatine analogue, β-guanidino propionic acid
(β-GPA), a well known inhibitor
of CreaT. Glyco-staining of CreaT, enriched by immuno-affinity
chromatography, mainly containing both the 70 and 55 kDa bands,
showed strong glycosylation of preferentially the upper 70 kDa
polypeptide indicating that the latter is a posttranslationally
modified form of the 55 kDa core protein. HeLa cells transfected with
rat CreaT cDNA showed an increase in [14C]-creatine uptake, when
compared to control cells, that was antagonized by β-GPA. In parallel, an increase in the expression of both the
70 and the 55 kDa polypeptides over endogenous CreaT of controls was
noticed on Western blots. Furthermore, we have found that chronic
creatine supplementation of rats, at very high dosage, down-regulates
in vivo the expression and/or accumulation of the CreaT in skeletal
muscle, but not in brain and heart [58]. Although the amounts of
creatine taken by athletes, 20 grams / day during a 10 days loading
phase and 5 grams as a maintenance dose during the following three
months (amounting to approximately 0.1 gram of Cr /kg body weight/
day), is significantly lower than the amounts given in the above
experiments to the rats (approximately 0.5 grams /kg body weight
/day), the finding made with laboratory animals nevertheless may have
consequences with respect to creatine supplementation schedules for
humans. In the future, however, detailed studies on humans are needed
to optimize the creatine supplementation schedules in use with
respect to the observed down-regulation of CreaT expression and/or
accumulation in animal experiments. According to most recent results,
using "normal" Cr supplementation schedules with humans,
CreaT seems also to be down-regulated, especially in combination with
exercise (Greenhaff et al. unpublished), but, over the time course of
this human trial, creatine transporter function did not seem to
become a limiting factor for maintaining normal intracellular
creatine levels. Nevertheless, as suggested earlier [86], a one month
pause, after three months of continuous creatine supplementation,
would still seem to be a reasonable thing to do.
With
respect to cardiac pathology, a down-regulation of creatine
transporter protein expression has recently been shown in
experimental animal models of heart disease, as well as in failing
human myocardium [91], indicating that the generally lowered PCr and
Cr levels measured in failing hearts are related to down-regulated
creatine transporter capacity. Thus, creatine supplementation, by
improving cellular energetics, may also turn out to be beneficial for
certain heart diseases.
Creatine supplementation as an adjuvant therapy for neuromuscular diseases:
Creatine
seems helpful not only for athletes to improve physical performance
on different levels (see above), but is also emerging as a
therapeutic aid for neuromuscular and neurodegenerative diseases
[85]. In some of these diseases, especially in mitochondrial
myopathies, a compensatory over-expression of Mi-CK, due to cellular
energy deficit, can lead to the formation of pathological
intramitochondrial crystalline Mi-CK inclusions [59], that, at least
in the β-GPA-animal model, disappear completely uponadministration of
creatine [60].
A protective effect of creatine on neuronal
function, especially during hypoxia or anoxia has been described
already some years ago first on brain slices [61,62]. Only recently,
encouraged by the success of creatine supplementation for improvement
of muscle performance in humans, have creatine and analogues
attracted new interest for brain metabolism [63,64,65]. In animal
models, creatine, as well as the creatine analog, β-GPA, was shown to
remarkably protect the brain of mice from hypoxic damage and seizures
in vivo [64,84] and significant neuroprotective effects of creatine
and cyclocreatine have been described in an animal model of
Huntington?s disease [44], as well as for Parkinsonism [66]. Creatine
and cyclocreatine afforded significant protection against malonate,
as well as 3-nitropropionic acid (3-NP) lesions and ROS generation in
the brain. Most recently, very remarkable neuro-protective effects
have been reported in an animal model of ALS, where 1% and 2%
creatine in the food significantly increased life span of FALS mice
in a dose-dependent manner and also delayed motor neuron degeneration
as measured by rotorod performance [85]. The observed neuroprotective
effects would be fully in line with the high expression levels and
the specific localizations of CK isoenzymes in brain, both regionally
[37] and on a cellular level [67], as well as functionally during
brain development and maturation [70] or in the adult brain
[68,69].
The above neuroprotective effects are paralleled
also with astonishing findings in transgenic mice expressing BB-CK in
liver, which normally is devoid of CK activity. Livers of such mice
become highly resistant to hypoxia [71] and liver toxins [72]. In
addition, CK and creatine, improving the intracellular
phosphorylation potential of these transgenic livers, confer
protection of ATP levels and stabilization of pH during a fructose
load [73]. Most recently, creatine supplementation of dystrophic
muscle cells from mdx mice was shown to result in a marked cell
protection, after a challenge by either hypo-osmotic swelling or high
extracellular [Ca2+], against chronically elevated calcium levels
seen in untreated control cells [74]. Promising preliminary results
and favourable subjective feed-back responses with patients suffering
from different neuromuscular diseases [75,86] have stimulated
controlled double-blinded clinical studies. Thus, the validity of
creatine supplementation as a possible adjuvant therapy for
neuromuscular and neurodegenerative diseases is currently being
tested. The first controlled clinical studies with patients have been
published [87,88] and some are about to appear [89,90], all showing a
rather positive outcome.
A bright future can be foreseen
for creatine as a nutritional supplement for healthy people, elderly
and reconvalescent, and for vegetarians on one hand [86], as well as
an adjuvant therapeutic aid for a plethora of new medical
applications [94]. Finally, for some cases, creatine and its
analogues will be used in the future for full-fletched pharmaceutical
intervention, e.g. for treating inborn errors of creatine metabolism
[76] or for anti-cancer therapy [77].
Acknowledgments:
This work was supported by the Swiss National Science Foundation, the Swiss Society for Muscle Diseases, the ETH-Zürich and privat sponsoring from Careal Holding, Benni &Co parents association Germany, Swiss Cancer Foundation, Innerschweizerische Krebsliga
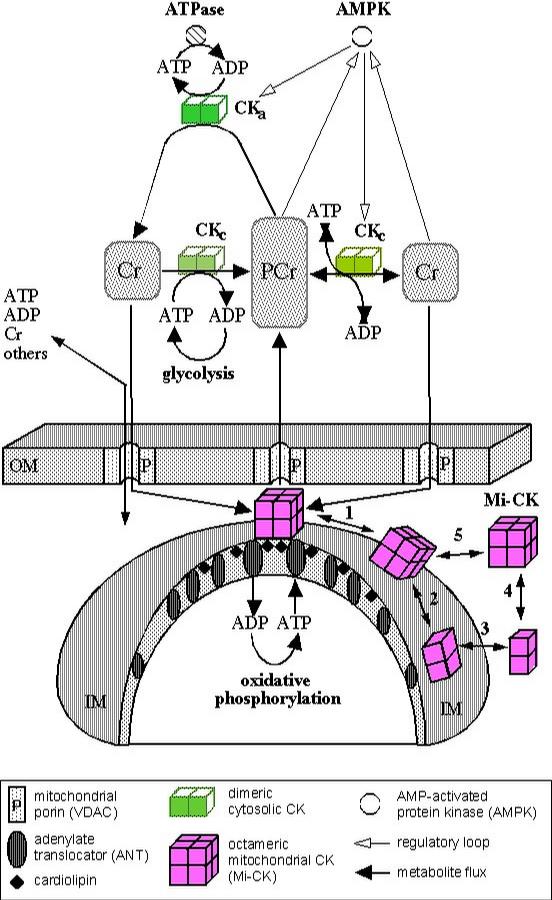
The PCr-circuit: a temporal and spatial energy buffering network and regulatory system for energy metabolism in cells with intermittently high energy requirements.
Upper, cytosolic side: the
bulk of soluble, cytosolic CK (CKc) equilibrates global ATP/ADP and
PCr/Cr ratios by its equilibrium reaction (depicted in the right
middle of the figure). In skeletal muscle at rest, these metabolite
levels are approximately 3-5 mM/10-20 µM and 20-40 mM/10-15 mM,
respectively (see [1,22,47]). One of the main functions of CKc is to
keep the concentration of free global ADP very low and thus to
maintaing global [ATP] remarkably stable also during cell activation.
This part of the PCr-circuit model represents the classical textbook
function of CK as a temporal energy buffer, being backed up by
adenylate kinase as a second safeguard against declining ATP and
rising ADP levels. Some of the cytosolic CKc is functionally coupled
to glycolysis and, during periods of anaerobic work-output and
recovery, preferentially accepts glycolytic ATP to replenish the very
large PCr pool (ATP from glycolysis, depicted in the left middle of
the figure). Additionally, however, some fractions of cytosolic CK,
are very specifically associated (CKa) with ATP requiring processes
at sites of energy consumption. For example, CKa is associated with
the contractile apparatus and the sarcoplasmic reticulum, where it
forms functionally coupled microcompartments with the acto-myosin
ATPase and the SR-Ca2+-ATPase, respectively, or with other ATP
requiring processes, like the Na+/K+-ATPase etc. (see top of figure).
There, ATP is directly regenerated in situ by CKa via PCr, thus
keeping local ATP/ADP ratios very high in the immediate vicinity of
these ATPases.
CK is phosphorylated
and down-regulated in its activity by AMP-dependent protein kinase
(AMPK, top right), which itself is the first enzyme that has been
found to be regulated by the PCr/Cr ratio, that is, AMPK is activated
by high creatine versus PCr levels [20].
Lower
mitochondrial side: mitochondrial Mi-CK is bound to the outer side of
the inner mitochondrial membrane (IM) and localized along the cristae
membranes, as well as at mitochondrial contact sites, where IM and OM
are in close vicinity [48]. At these sites, Mi-CK octamers are
forming microcompartments with porin (P) and adenine nucleotide
translocase (ANT) for energy transfer from ATP to Cr, followed by
vectorial transport of PCr into the cytosol. ATP generated by
oxidative phosphorylation is preferentially accepted by Mi-CK
octamers, transphosphorylated onto Cr, which is entering through
mitochondrial porin (P, or VDAC), to give PCr which then is exported
into the cytosol. Thus, under high work-load, PCr would be shuttled
from mitochondria to sites of energy consumption (ATPases, top of
figure), where it is then used
by CKa to
regenerate ATP locally in situ to fuel these ATP-requiring processes
and to keep local ATP/ADP ratios very high. Cr would diffuse back to
the mitochondria to be recharged again. This part of the model
represents the spatial buffering function of the PCr-circuit. In this
model, the specifically localized CK isoenzymes at sites of energy
consumption and energy production are connected via PCr and Cr as
mediators, generating metabolic waves and dampening oscillations of
metabolites [22,46].
The dynamic
recruitment of either free or membrane-bound Mi-CK octamers
(double-arrows 5 or 1, respectively), possibly depending on the
metabolic state of the mitochondria, the dynamic octamer/dimer
equilibrium of Mi-CK (double arrows 2 and 4), as well as
octamerization of Mi-CK dimers bound on the IM (double-arrow 2), all
observed in vitro, are schematically visualized as potential
modulatory events for long-term metabolic regulation. The interaction
of Mi-CK with porin and complex formation of the enzyme with ANT,
most likely facilitated by cardiolipin associated with ANT, are also
illustrated. Under the conditions expected to prevail in the
mitochondrial intermembrane space, however, the equilibria of these
reactions, as observed in vitro, would clearly favour the
membrane-bound octamer [21,25]. However,since the formation of
contact sites and the establishment of the protein complexes are
thought to be rather dynamic, a on/off recruitment of Mi-CK octamer
into contact sites could easily be envisaged. Finally, these events
that are heavily influenced by the exquisite sensitivity of Mi-CK
towards peroxynitrite and other ROS [26], may be relevant also for
the control of the permeability transition pore [39-41, 45].
Figure1:
References:
[1] Wallimann, T.,
Wyss, M., Brdiczka, D., Nicolay, K.,and H. M. Eppenberger (1992).
Intracellular compartmentation, structure and function of creatine
kinase isoenzymes in tissues with high and fluctuating energy
demands: the "PCr-circuit" for cellular energy homeostasis.
Biochem. J. 281, 21-40.
[2]
McFarland, E.W., Kushmerick, M.J., and T. Moerland (1994). Activity
of creatine kinase in a contracting mammalian muscle of uniform fiber
type. Biophys. J. 67, 1912-1924.
[3] Wiseman, R.W., and M. Kushmerick (1995). Creatine kinase
equilibrium follows solution thermodynamics in skeletal muscle:
31P-NMR studies using creatine analogs. J. Biol. Chem. 270,
12428-12438.
[4] Wallimann, T.
(1994). 31P-NMR-measured creatine kinase reaction flux in muscle: a
CAVEAT! J. Muscle Res. Cell Motil. 17, 177-181.
[5] VanDeursen, J., Ruitenbeek, W., Heerschap, A.,
Jap, P., terLaak, H., and B. Wieringa (1994). Creatine kinase in
skeletal muscle energy metabolism: a study of mouse mutants with
graded reduction in muscle CK expression. Proc. Natl. Acad. Sci. USA
91, 9091-9095.
[6] Wallimann, T.
(1994). Dissecting the role of creatine kinase. Current Biology 1,
42-46.
[7] Kreis, R., Koster,
M., Kamber, M., Hoppeler, H., and C. Boesch (1997). Peak assignment
in localized 1H MR spectra of human muscle based on oral creatine
supplementation. Magn. Res. in Med. 37, 159-163.
[8] LeRumeur, E., LeTallec, N., Kernec, F., and
J.D. deCertaines (1997). Kinetics of ATP to ADP b-phosphoryl
conversion in contracting skeletal muscle by in vivo 31P-NMR
magnetization transfer. NMR in Biomed. 10, 67-72.
[9] Ntziachristos, V., Kreis, R., Boesch, C., and
B. Quistorff (1997). Dipolar resonance frequency shifts in 1H MR
spectra of skeletal muscle: confirmation in rats at 4,7 T in vivo and
observation of changes postmortem. Magn. Reson. Med. 38, 33-39.
[10] Williams, J.P., and J.P. Headrick (1996).
Differences in nucleotide compartmentation and energy state in
isolated and in sit rat heart: assessment by 31 P-NMR spectroscopy.
Biochim. Biophys. Acta 1276, 71-79.
[11] Hochachka, P.W., and M.K. Mossey (1998). Does muscle creatine
phosphokinase have access to the total pool of phosphocreatine plus
creatine? Am. J. Physiol. 274, R868-872.
[12] VanDorsten, F., Wyss, M., Wallimann, T., and K. Nicolay (1997).
Activation of sea urchin sperm motility is accompanied by an increase
in the creatine kinase exchange flux. Biochem. J. 325, 411-416.
[13] Kaldis, P., Kamp, G., Piendl, T., and T.
Wallimann (1997). Functions of creatine kinase isoenzymes in
spermatozoa. Adv. in Develop. Biology 5, 275-312.
[14] Steeghs, K., Benders, Ad., Oerlemans F.
deHaan, A., Heerschap, A., Ruitenbeek, W., Jost, C., van Deursen, J.,
Peryman, B., Pette, D., Brückwilder, M., Koudijs, J., Jap, P.,
Veerkamp, J., and B. Wieringa (1997). Altered Ca2+-response in
muscles with combined mitochondrial and cytosolic creatine kinase
deficiencies. Cell 89, 93-103.
[15] Rossi, A.M., Eppenberger, H.M., Volpe, P., Cotrufo, R., and T.
Wallimann (1990). Muscle type MM-creatine kinase is specifically
bound to sarcoplasmic reticulum and can support Ca2+-uptake and
regulate local ATP/ADP ratios. J. Biol. Chem. 265, 5258-5266.
[16] Korge, P., and K.B. Campbell (1994). Local
ATP regeneration is important for sarcoplasmic reticulum Ca2+-pump
function. Am. J. Physiol. 267, C357-366.
[17] Minajeva, A., Ventura-Clapier, R., and V. Veksler (1996).
Ca2+-uptake by cardiac sarcoplasmic reticulum ATPase in situ strongly
depends on bound creatine kinase. Pflügers Arch. 432, 904-912.
[18] Stolz M., and T. Wallimann (1998).
Myofibrillar interaction of cytosolic creatine kinase (CK)
isoenzymes: allocation of N-terminal binding epitope in MM-CK and
BB-CK. J. Cell Sci. 111, 1207-1216.
[19] Kraft, Th., Nier, V., Brenner, B., and T. Wallimann (1996).
Binding of creatine kinase to the I-band of skinned skeletal muscle
fibers is mediated by glycolytic enzymes: an in situ biochemical
approach. Biophys. J. 70, A292.
[20] Ponticos, M., Lu, Q.L., Morgan, J.E., Hardie, D.G., Partridge,
T.A., and D. Carling (1998). Dual regulation of AMP-activated protein
kinase provides a novel mechanism for the control of creatine kinase
in skeletal muscle. EMBO J. 17, 1688-1699.
[21] Schlattner, U., Forstner, M., Eder, M., Stachowiak, O.,
Fritz-Wolf, K., and T. Wallimann (1998). Functional aspects of the
X-ray structure of mitochondrial creatine kinase: a molecular
physiology approach. Mol. Cell Biochem. 184, 125-140.
[22] Wyss, M., Smeitink, J., Wevers, R., and T.
Wallimann (1992). Mitochondrial creatine kinase: a key enzyme of
aerobic energy metabolism. Biochim. Biophys. Acta 1102, 119-166.
[23] Brdiczka, D., Kaldis, P., and T. Wallimann
(1994). In vitro complex formation between the octamer of
mitochondrial creatine kinase and porin. J.Biol. Chem. 269,
27640-27644.
[24] Fritz-Wolf,
K., Schnyder, T., Wallimann, T., and W. Kabsch (1996). Structure of
mitochondrial creatine kinase. Nature 381, 341-345.
[25] Stachowiak, O., Schlattner, U., Dolder, M.,
and T. Wallimann (1998). Oligomeric state and membrane binding
behaviour of creatine kinase isoenzymes: implications for cellular
function and mitochondrial structure. Mol. Cell Biochem. 184,
141-151.
[26] Stachowiak, O.,
Dolder, M., Wallimann, T., and Ch. Richter (1998). Mitochondrial
creatine kinase is a prime target of peroxynitrite-induced
modification and inactivation. J. Biol. Chem. 273, 16694-16699.
[27] Ghafourifar, P., and C. Richter (1997).
Nitric oxide synthase activity in mitochondria. FEBS Lett. 418,
291-296.
[28] Gross, W.L., Bak,
M.I., Ingwall, J.S., Arstall, M.A., Smith, T.W., Balligand, J.L., and
R.A. Kelly (1996). Nitric oxide inhibits creatine kinase and
regulates rat heart contractile reserve. Proc. Natl. Acad. Sci.
U.S.A. 93, 5604-5609.
[29]
Adams, V., Yu, J., Möbius-Winkler, S., Linke, A., Weigl, C.,
Hilbrich, L., Schuler, G., and R. Hambrecht (1997). Increased
inducible nitric oxide synthase in skeletal muscle biopsies from
patients with chronic heart failure. Biochem. Mol. Medicine 61,
152-160.
[30] Joe, E.K.,
Schussheim, A.E., Longrois, D., Maki, T., Kelly, R.A., Smith, T.W.,
and J.L. Balligand (1998). Regulation of cardiac myocyte contractile
function by inducible nitric oxide synthase (iNOS): mechanisms of
contractile depression by nitric oxide. J. Mol. Cell Cardiol. 30,
303-315.
[31] Wendt, S.,
Stachowiak, O., Dolder, M., Schlattner, U., and T. Wallimann (1998).
Effects of peroxynitrite on creatine kinase: implications for
Ca2+-handling and apoptosis. 5th Internatl. Symp. on Guanidino
Compounds in Biology and Medicine (Yokohama, Japan, Sept. 2-3, 1998),
Abstract S3-7, pp 63.
[32]
Mekhfi, H., Veksler, V., Mateo, Ph. Maupoil, V., Rochette, L., and R.
Ventura-Clapier (1996). Creatine kinase is the main target of
reactive oxygen species in cardiac myofibrils. Circ. Res. 78,
1016-1027.
[33] Konorev, E.A.,
Hogg, N., and B. Kalyanaraman (1998). Rapid and irreversible
inhibition of creatine kinase by peroxynitrite. FEBS Lett 427,
171-174.
[34] Dykens, J.A.
(1994). Isolated cerebral and cerebellar mitochondria produce free
radicals when exposed to elevated Ca2+ and Na+: implications for
neurodegeneration. J. Neurochem. 63, 584-591.
[35] Molkentin, J.D., Lu, J.R., Antos, C.L., Markham, B., Richardson,
J., Robbins, J., Grant, S.R., and E.N. Olson (1998). A
calcineurin-dependent transcriptional pathway for cardiac
hypertrophy. Cell 93, 215-228.
[36] Mattson, M.P.
(1992). Calcium as sculptor and destroyer of neural circuitry. Exp.
Gerontol. 27, 29-49.
[37]
Kaldis, P., Hemmer, W., Zanolla, E., Holtzman, D., and T. Wallimann
(1996). Hot spots of creatine kinase localization in brain:
cerebellum, hippocampus and choroid plexus. Dev. Neurosci. 18,
542-554.
[38] David, S.,
Shoemaker, M., and B.E. Haley (1998). Abnormal properties of creatine
kinase in Alzheimer?s disease brain: correlation of reduced enzyme
activity and active site photolabeling with aberrant cytosol-membrane
partitioning. Mol. Brain Res. 54, 276-287.
[39] Beutner, G., Rück, A., Riede, B., Welte, W., and D.
Brdiczka (1996). Complexes between kinases, mitochondrial porin and
adenylate translocator in rat brain resemble the permeability
transition pore. FEBS Letters 396, 189-195.
[40] Beutner, G., Rück, A., Riede, B., and D. Brdiczka (1998).
Complexes between porin, hexokinase, mitochondrial creatine kinase
and adenylate translocator display properties of the permeability
transition pore. Implication for regulation of permeability
transition by the kinases. Biochim. Biophys. Acta 1368, 7-18.
[41] 0?Gorman, E., Beutner, G., Dolder, M.,
Koretsky, A.P., Brdiczka, D., and T. Wallimann (1997). The role of
creatine kinase in inhibition of mitochondrial permeability
transition. FEBS Letters 414, 253-257.
[42] Crompton, M., Ellinger, H., and A. Costi (1988). Inhibition by
cyclosporin A of a Ca2+-dependent pore in heart mitochondria
activated by inorganic phosphate and oxidative stress. Biochem. J.
255, 357-360.
[43] Holtzman, D.,
and T. Kekelidze (1998). Guanidino analogues and the brain creatine
kinase system. 5th Internatl. Symp. on Guanidino Compounds in Biology
and Medicine (Yokohama, Japan, Sept. 2-3, 1998), Abstract SL-1, pp
22.
[44] Matthews R.T., Yang,
L., Jenkins, B.G., Ferrante, R.J., Rosen, B.R., Kaddurah-Daouk, R.,
and M.F. Beal (1998). Neuroprotective effects of creatine and
cyclocreatine in animal models of Huntington?s disease. J. Neurosci.
18, 156-163.
[45] Rück, A.,
Dolder , M., Wallimann, T., and D. Brdiczka (1998). Reconstituted
adenine nucleotide translocase forms a channel for small molecules
comparable to the mitochondrial permeability transition pore. FEBS
Lett. 426, 97-101.
[46] Wyss,
M., and T. Wallimann (1992). Metabolite channelling in aerobic energy
metabolism. J. theor. Biol. 158, 129-132.
[47] Saks, V.A., Khuchua, Z.A., Vasilyeva, E.V., Belikova, O.Yu., and
A.V. Kuznetsov (1994). Metabolic compartmentation and substrate
channelling in muscle cells. Role of coupled creatine kinases in in
vivo regulation of cellular respiration - a synthesis. Mol. Cell
Biochem. 133/134, 155-192.
[48]
Brdiczka, D., Beutner, G., Rück, A., Dolder, M., and T.
Wallimann (1998). The molecular structure of mitochondrial contact
sites. Their role in regulation of energy metabolism and permeability
transition. BioFactors 8, 235-242.
[49] Leist, M. and P. Nicotera (1998). Apoptosis, excitotoxicity and
neuropathology. Exp. Cell Res. 239, 183-201.
[50] Wyss, M., and T. Wallimann (1994). Creatine metabolism and the
consequencesof creatine depletion in muscle. Mol.Cell Biochem.
133/134, 51-66.
[51]
Guerrero-Ontiveros, L. and T. Wallimann (1998). Creatine
supplementation in health and disease. Effects of chronic creatine
ingestion in vivo: down-regulation of the expression of creatine
transporter isoforms in skeletal muscle. Mol. Cell Biochem. 184,
427-437.
[52] Greenhaff, P.L.
Casey, A., Short, A.H., Harris, R., Soderlund, K. and E. Hultman
(1993). Influence of oral creatine suplementation on muscle torque
during repeated bouts of maximal voluntary exercise in man. Clin.
Sci. 84, 565-571.
[53] Balsom,
P.D., Soderlund, K., and B. Ekblom (1994). Creatine in humans with
special reference to creatine supplementation. Sports Med. 18,
268-280.
[54] Greenhaff, P.L.,
Bodin, K., Soderlund, K., and E. Hultman (1994). Effect of oral
creatine supplementation on skeletal muscle phosphocreatine
resynthesis. Am. J. Physiol. 266, E725-E730.
[55] Hultman, E., Soderlund, K., Timmons, J.A., Cederblad, G., and
P.L. Greenhaff (1996). Muscle creatine loading in man. Am J. Appl.
Physiol. 81, 232-237.
[56]
Greenhaff, P.L. (1997). The nutritional biochemistry of creatine.
Nutritional Biochem. 8, 610-618.
[57] Brönnimann, M., Accola, C., Strub, W., Villiger, B., and T.
Wallimann (1998). Beneficial effect of creatine supplementation for
high-intensity endurance performance. 5th Internatl. Symp. on
Guanidino Compounds in Biology and Medicine (Yokohama, Japan, Sept.
2-3, 1998), Abstract S1-3, pp 32.
[58] Guerrero, L.M., Walzel, B., and T. Wallimann (1998). Creatine
transporter polypeptides are down-regulated by chronic creatine
supplementation. 5th Internatl. Symp. on Guanidino Compounds in
Biology and Medicine (Yokohama, Japan, Sept. 2-3, 1998), Abstract
S1-8, pp 37.
[59] Stadhouders,
Ad.M., Jap, P., Winkler, H.P., Eppenberger, H.M., and T. Wallimann
(1994). Mitochondrial creatine kinase: a major constituent of
pathological inclusions seen in mitochondrial myopathies. Proc. Acad.
Sci. U.S.A. 91, 5089-5093.
[60]
O?Gorman, E., Fuchs, K-H., Tittmann, P., Gross, H., and T. Wallimann
(1997). Crystalline mitochondrial inclusion bodies isolated from
creatine-depleted rat soleus muscle. J. Cell Sci. 110,
1403-1411.
[61] Woznicki, D.T.,
and J.B. Walker (1980). Utilization of cyclocreatin phosphate, an
analogue of creatine phosphate, by mouse brain during ischemia and
its sparing action on brain energy reserves. J. Neurochem. 35,
1247-1253.
[62] Whittingham,
T.S., and P. Lipton (1981). Cerebral synaptic transmission during
anoxia is protected by creatine. J. Neurochem. 37, 1618-1621.
[63] Carter, A.J., Müller, E., Pschorn, U.,
and W. Stransky (1995). Preincubation with creatine enhances levels
of creatine phosphate and prevents anoxic damage in rat hippocampus
slices. J. Neurochem. 64, 2691-2699.
[64] Holtzman, D., Meyers, R., O?Gorman, E., Khait, I., Wallimann,
T., Allred, E., and F. Jensen (1997). In vivo brain phosphocreatine
and ATP regulation in mice fed a creatine analog. Am. J. Physiol.
272, C1567-C1577.
[65] Wilken,
B., Ramires, J.M., Probst, I., Richter, D.W., and F. Hanefeld (1998).
Creatine protects the central respiratory network of mammals under
anoxic conditions. Pediatric Res. 43, 8-14.
[66] Matthews, R.T., Ferrante, L.C., Klinvenyi, P., Yang, L.C.,
Klein, A.M., Mueller. G., Kaddurah-Daouk, R., and M.F. Beal (1999).
Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp. Neurol.
157, 142-149.
[67] Hemmer, W.,
Zanolla, E., Furter-Graves, E., Eppenberger, H.M., and T. Wallimann
(1994). Creatine kinase isoenzymes in chicken cerebellum: specific
localization of brain-type CK in Bergmann glial cells and muscle-type
CK in Purkinje neurons. Eur. J Neurosci. 6, 538-549.
[68] Hemmer, W., and T. Wallimann (1993).
Functional aspects of creatine kinase in brain. Dev. Neurosci. 15,
249-260.
[69] Hemmer, W., and T.
Wallimann (1994). Creatine kinase in non-muscle tissues and cells.
Mol. Cell. Biochem. 133/134, 193-220.
[70] Holtzman, D., Tsuji, M., Wallimann, T., and W. Hemmer (1993).
Functional maturation of creatine kinase in rat brain. Dev. Neurosci.
15, 261-270.
[71] Miller, K.,
Halow, J., and A.P. Koretsky (1993). Phosphocreatine protects
transgenic mouse liver expressing creatine kinase from hypoxia and
ischemia. Am. J. Physiol. 265, C1544-C1551.
[72] Hatano, E., Tanaka, A., Iwata, S., Satoh, S., Kitai, T.,
Tsunekawa, S., Inomoto, B., and Y. Yamaoka (1996). Induction of
endotoxin tolerance in transgenic mouse liver expressing creatine
kinase. Hepatology 24, 663-639.
[73] Brosnan, J.M., Chen, L., Wheeler, C.E., vanDyke, T., and A.P.
Koretsky (1991). Phosphocreatine protects ATP from a fructose load in
transgenic mouse liver expressing creatine kinase. Am J. Physiol.
C1191-C1200.
[74] Pulido, S. M.,
Passaquin, A.C., Leijendekker W. J., Wallimann, T. and U.T.
Rüegg (1998). Creatine supplementation improves intracellular
calcium handling and survival in mdx skeletal muscle cell. FEBS
Letters 439, 357-362.
[75]
Brönnimann, M., and T. Wallimann (1997). Creatine: Break-through
for the treatment of neuromuscular disorders? Swiss Soc. for Muscle
Diseases. Mitteilungsblatt 43, 3-10.
[76] Stöckler, S., Hanefeld, F., and J. Frahm (1996). Creatine
replacement therapy in guanidinoacetate methyltransferase deficiency,
a novel inborn error of metabolism. Lancet 348, 789-790.
[77] Martin, K.J., Winslow, E.R., O?Keefe, M.,
Khandekar, V.S., Hamlin, A., Lillie, J.W., and R. Kaddurah-Daouk
(1996). Specific targeting of tumor cells by the creatine analog
cyclocreatine. Internatl. J. Oncol. 9, 993-999.
[78] Shoubridge, E.A., Bland, J.L., and G.K. Radda
(1984). Regulation of creatine kinase during steady-state isometric
twitch contraction in rat skeletal muscle. Biochim. Biophys. Acta
805. 72-78.
[79] Goudemant,
J.F., Francaux, M., Mottet, I., Demeure, R., Sibomana, M., and X.
Sturbois (1997). 31P-NMR saturation transfer study of the creatine
kinase reaction in human skeletal muscle at rest and during exercise.
Magn. Res. in Medicine 37, 744-753.
[80] Hornemann, Th., Stolz, M., and T. Wallimann (1999). Interaction
of muscle-type creatine kinase (MM-CK) isoform with the myofibrillar
M-band is mediated by four lysine residues located at the N-terminus.
J. Muscle Res. Cell Motil. 20, 112 abstract (paper submitted)
[81] Soboll, S., Brdiczka, D., Jahnke, D.,
Schulze, K., Schmidt, A., Schlattner, U., Wendt, S., and T. Wallimann
(1999). Octamer-dimer transitions of mitochondrial creatine kinase in
heart disease. J. Mol. Cell Cardiol. 31, 857-866
[83] van Leemputte, M., Vandenberghe, K., and P.
Hespel (1999). Shortening of muscle relaxation time after creatine
loading. J. Appl. Physiol. 86, 840-844.
[84] Holtzman D., Togliatti, A., Khait, I., and F. Jensen (1998).
Creatine increases survival and suppresses seizures in the hypoxic
immature rat. Pediatr. Res. 44, 410-414.
[85] Klivenyi, P., Farrante, R.J., Matthews, R.T., Bogsdanov, M.B.,
Klein, A.M., Andreassen, O.A., Mueller, G., Wermer, M.,
Kadurah-Daouk, R., and F.M. Beal (1999). Neuroprotective effects of
creatine in a transgenic animal model of amyotrophic lateral
sclerosis. Nature Medicine 5, 347-350.
[86] Wallimann, T. (1999) Creatine supplementation: positive effects
in health and disease. German Muscle Report (Deutsche Gesellschaft
für Muskelkranke, DGM) Vol 2, 25 -30; ibid. German Muscle Report
Vol 3, 2-3. (ISSN 0178-0352).
[87] Tarnopolsky, M., Roy, B.D., and J.R. MacDonald (1997).
Randomized, controlled trial of creatine monohydrate in patients with
mitochondrial cytopathies. Muscle and Nerve 20, 1502-1509.
88] Tarnopolsky, M., and J. Martin (1999).
Creatine monohydrate increases strength in patients with
neuromuscular disease. Neurology 52, 854-857.
[89] Klopstock, T., Schlamp, V.,
Schmidt, F., Gekler,
F., Hartard, M., Pongratz,
D., Walter, M., Gasser, T.,
Straube, A.,
Dietrich, M., and W. Müller-Felber
(1999).
Creatine monohydrate in mitochondrial
diseases: a
double-blind, placebo-controlled,
cross-over
study in 16 patients with chronic
progressive
external opthalmoplegia or mitochondrial
mypathies. Neurology 52, Suppl. 2, A543-544.
[90] Walter, M.C., Lochmüller,
H., Hartard, M.,
Reilich, P., Pongratz, D., and
W. Müller-Felber
(1999). Creatine
monohydrate in muscular dystrophies:
a
double-blind, placebo-controlled clinical study.
Neurology, Suppl. 2, A543-544.
[91] Neubauer, S., Remkes, H., Spindler,
M., Horn, M.,
Prestle, J., Walzel, B., Ertl, G.,
Hasenfuss, G., and
T. Wallimann (1999).
Downregulation of the
Na+-creatine cotransporter
in failing human myocardium
and in experimental
heart failure. Circulation (in
press, 1999).
[92] Volek, J.S.,
Duncan, N.D., Mazzetti,
S.A., Staron, R.S., Putukian,
M., Gomez, A.L.,
Pearson, D.R., Fink, W. J., and W.J.
Kraemer
(1999). Performance and muscle fiber
adaptations
to creatine supplementation and heavy
resistance
training. Med. Sci. Sports Exerc. 31,
1147-1156.
[93] Duke, A.M., and
D.S. Steele (1999).
Effects of creatine phosphate on
calcium
regulation by the sarcoplasmic reticulum in
mechanically skinned rat skeletal fibres. J.
Physiol.
517, 447-458.
[94] Vandenberghe,
K., Goris, M., Van
Hecke, P., Van Leemputte, M.,
Vangerven, L., and
P. Hespel (1997). Long-term
creatine intake is
beneficial to muscle performance
during
resistance training. J. Appl. Physiol. 83,
2055-2063.
[95] Wallimann, T.,
Schlattner, U.,
Guerrero, L., and M. Dolder (1999) The
phosphocreatine circuit and creatine
supplementation, both come of age! In: Guanidino
Compounds in Biology and Medicine (Mori, A.,
Ishida,
M., and Clark, J.F., eds.) Blackwell
Science Asia Pry
Ltd. pp 117-129.
[96] Robinson,
T.M., Sewell, D.,A., Hultman, E., and P. Greenhaff (1999) Role of
submaximal exercise in promoting creatine and glycogen accumulation
in human skeletal muscle. J. Appl. Physiol. 87, 598-604